RESEARCH
Organic semiconducting polymers
More than 20 years have passed since Professor Shirakawa received the Nobel Prize in Chemistry for his achievements in the discovery and application of conductive polymers. It was in the period following this award that major progress was made in the research field of organic semiconducting polymers. Compared to the doping of simple polyacetylene, a polymer with alternating carbon-carbon single and double bonds, the chemical structure of polymers has become more and more complex. In our group, we synthesize polymers by the increasingly prevalent polycondensation method between two or more monomers using a Pd-catalyzed cross-coupling reaction. The resulting polymers feature narrow band gaps and high charge carrier mobilities.
Similar to inorganic semiconductors, there are two types of charge carriers in organic semiconducting polymers. Those that transport holes are called p-type semiconducting polymers, while those that transport electrons are called n-type semiconducting polymers. P-type organic semiconducting polymers are common whereas the library of n-type organic semiconducting polymers is lacking because there are not many useful building blocks for electron-deficient polymers – they face instability or tough synthesis routes. Our main target is thus high mobility n-type semiconducting polymers. For example, benzothiadiazole (BT) is a promising acceptor unit due to the electron-deficient structure allowing it to stabilize the transport of electrons. By extension of the aromatic unit and inclusion of additional heteroatoms, the electron-accepting character of the unit is increased as benzobisthiadiazole (BBT) is formed. However, this presents one of the major challenges: solubility issues in common/industrial organic solvents which limits processing. This requires the introduction of alkyl chains into the adjacent aromatic rings of BBT. These alkyl chains cause a significant twist of the main chain polymer backbones and accordingly result in the decreased crystallinity and performance. In order to overcome this problem, thiadiazolobenzotriazole (TBZ) was developed. As an alkyl group can be introduced into the nitrogen moiety of TBZ, sufficient solubilities and a planar main chain backbone of resulting polymers can be achieved. As a result, TBZ-based n-type semiconducting polymers displayed remarkably high electron mobilities in transistors.
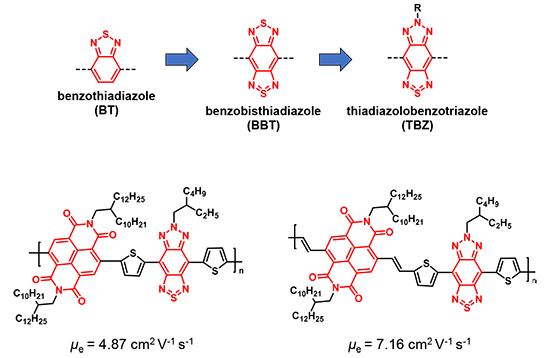
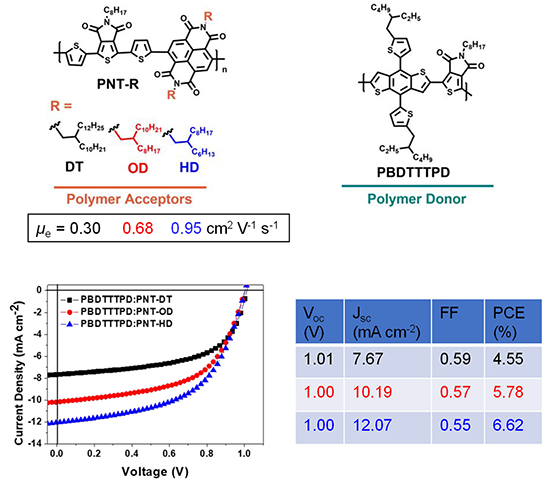
Conventional organic solar cells are composed of p-type semiconducting polymers and small molecule n-type semiconductors, but all-polymer solar cells have recently attracted significant attention due to the synthetic accessibility of various n-type semiconducting polymers, easy fabrication and long-term stability of the devices. Our n-type semiconducting polymers were applied to all-polymer solar cells, and their photovoltaic performances were evaluated. As the alkyl side chain length decreased, the electron mobility and photovoltaic properties increased.
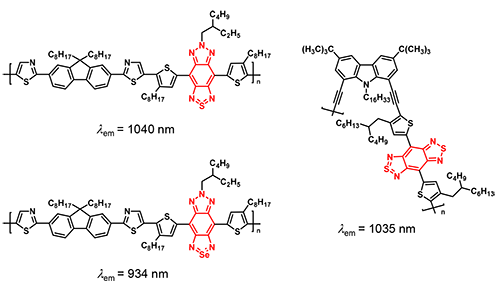
Triply-fused aromatic ring structures such as BBT and TBZ show near-infrared emission due to the narrow band gaps. Nanoparticles of BBT and TBZ-based semiconducting polymers have been prepared and are currently under investigation for bio-imaging.
Donor-acceptor chromophores by [2+2] cycloaddition-retroelectrocyclization
[2+2] Cycloaddition-retroelectrocyclization (CA-RE) of electron-rich alkynes and electron-deficient alkenes produce nonplanar donor-acceptor chromophores. When electronically appropriate substituents are employed, the CA-RE reaction rapidly proceeds under mild conditions in quantitative yields owing to the pericyclic nature of the reaction, suggesting potential for a new easily employable click chemistry reaction. Donor-acceptor molecules show many interesting properties including large optical nonlinearity, potent redox activities, and ion recognition. We found that the frontier molecular orbital energy levels can be tuned by adding electron-deficient alkene molecules such as tetracyanoethylene (TCNE) and 7,7,8,8-tetracyanoquinodimethane (TCNQ) to the electron-rich alkynes of p-type semiconducting polymers. This synthetic approach produced new block copolymer compatibilizers with desired energy levels, which could improve photovoltaic properties of organic solar cells.

Lignin-derived biomass polymers
Pollution of marine environment by petroleum-derived plastics has become a major global issue. In order to solve this problem, we are developing biodegradable thermoplastic biopolymers from lignin, a woody biomass. Lignin makes up 20-30 weight percent of wood, though this is conventionally combusted and used as an energy source. This is a great loss of a useful resource.
We use the dicarboxylic acid derivative 2-pyrone-4,6-dicarboxylic acid (PDC), which is obtained from the metabolic process of lignin, as a monomer in the polymerization. PDC polyesters have been successfully synthesized by polycondensation of PDC with diol comonomers. The resulting PDC polyesters displayed excellent thermal and mechanical properties due to the pseudo-aromatic pyrone rings in the main chain. Initial tests of PDC materials suggest potential use for applications such as ductile plastics and elastomers. As the PDC polyesters are biodegradable, they are environmentally friendly materials.

